EXHIBIT 99.1
Published on January 13, 2020
EXHIBIT 99.1
Acasti Pharma Reports Topline Results for TRILOGY 1 Phase 3 Trial of CaPre
Reports 30.5% and 36.7% reduction in triglyceride levels, compared with baseline, among patients receiving CaPre at 12 and 26 weeks respectively, as well as 42.2% reduction in triglyceride levels among patients receiving CaPre while on background statin therapy at 12 weeks
Despite positive results in CaPre arm, study did not reach statistical significance
due to unusually large placebo effect; further analysis is underway
No treatment-related serious adverse events reported in the trial
The last patient completed their final visit in the TRILOGY 2 trial late last week,
topline results expected now by mid-February 2020
LAVAL, Québec, Jan. 13, 2020 (GLOBE NEWSWIRE) -- Acasti Pharma Inc. (“Acasti or the “Company”) (NASDAQ: ACST – TSX-V: ACST), a biopharmaceutical innovator focused on the research, development and commercialization of its prescription drug candidate CaPre® (omega-3 phospholipid) for the treatment of severe hypertriglyceridemia (triglyceride blood levels from 500 mg/dL to 1500 mg/dL), today announced topline results for the Primary Endpoint (triglyceride reduction at 12 and 26 weeks) from its Phase 3 TRILOGY 1 trial for the Company's lead product candidate, CaPre.
The Company reported a 30.5% median reduction in triglyceride levels among all patients receiving CaPre, compared to a 27.5% median reduction in triglyceride levels among patients receiving placebo at 12 weeks. The Company also reported a 42.2% median reduction in triglycerides among patients receiving CaPre while on background statin therapy at 12 weeks, compared to a 31.5% median reduction in triglyceride levels among patients receiving placebo and on background statin therapy. In addition, the Company reported a 36.7% median reduction in triglyceride levels among patients receiving CaPre at 26 weeks (end of the study), compared to a 28.0% median reduction in triglyceride levels among patients receiving placebo. Both the placebo and CaPre study groups experienced significant reductions in triglycerides within the first four weeks from baseline, and even though the difference at 12 and 26 weeks was in favor of CaPre, due to the unexpectedly large placebo response, TRILOGY 1 did not reach statistical significance. The safety profile of CaPre in TRILOGY 1 was similar to placebo, as there were no significant difference in treatment-related serious adverse events in the trial. Results for all of the secondary and exploratory endpoints were delayed as previously reported on December 23, 2019, and are expected to be available by the end of Q1, 2020.
The observed reductions in triglyceride levels in the placebo group were far greater than that seen in any previous triglyceride lowering trial with a prescription omega-3. The placebo used in the TRILOGY trials is simple cornstarch, which is a complex carbohydrate with a low glycemic index, and consequently would be expected to have a neutral effect on key biomarkers of patients in the placebo group. In similar previously conducted triglyceride lowering trials involving prescription omega-3 preparations, the placebo responses (using corn oil, olive oil, or vegetable oil) ranged from a change of +16% to -17% across 18 interventions arms, with 14 of 18 arms ranging between +10% to -10%.
A table summarizing the placebo and active triglyceride lowering results from all of these previous hypertriglyceridemia trials is presented below:
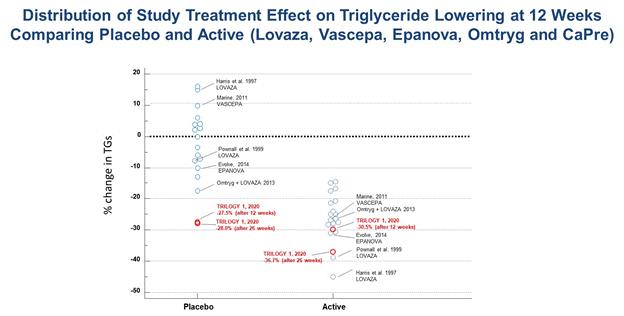
Given that cornstarch is likely not the root cause for the significant placebo response in TRILOGY 1, the Company is carefully evaluating other possible explanations. The Company has noted that a high placebo response at 5 sites (out of a total of 54 enrolling sites) disproportionately contributed to the overall placebo response, and is being further investigated. A full audit of these sites, including review of all raw data and records from patients taking both CaPre and placebo, will be conducted to identify a possible root cause of the unprecedented placebo effect. This audit is likely to take at least several weeks, with an outcome expected by the end of February 2020. Additional avenues of investigation will include further assessment related to specific continuation or discontinuation of other lipid lowering drugs during screening, and changes in the use of other lipid-lowering medications during the trial. Furthermore, the results of the ongoing TRILOGY 2 trial with CaPre may provide additional important information and insight in this regard. If one or more of these investigations provide a plausible explanation as to what may have influenced the placebo arm, and assuming the primary endpoint reaches statistical significance in TRILOGY 2, the Company may still have a path forward to file an NDA, and would seek a meeting with the FDA as soon as possible to discuss all of the TRILOGY data, investigational findings, and obtain their input and guidance on next steps.
Dariush Mozaffarian, M.D., Dr.P.H., principal investigator for the study, commented, “Compared with baseline, triglyceride levels in subjects receiving CaPre were substantially lower at 12 and 26 weeks in the CaPre arm, with 30.5% and 37.5% lower levels, respectively. However, these reductions were accompanied by larger than expected declines in triglyceride levels in the placebo group, which remain unexplained and highly unusual. Initial analyses suggest no protocol deviations in treatment allocation, capsule contents, laboratory quality control, or mismatched randomization that could explain these highly unusual placebo results. We are continuing to evaluate the data in detail to assess possible explanations. I am also hopeful that TRILOGY 2 topline data, expected in late January 2020, may provide more insight into this unprecedented placebo response seen in TRILOGY 1.”
Jan D’Alvise, president and CEO of Acasti Pharma, stated, “First, we wish to thank the physicians, study professionals, and of course the 242 patients who dedicated their time to participate in this trial. While we are encouraged by the magnitude of reduction in triglyceride levels seen among patients receiving CaPre, the large placebo effect was completely unexpected, and was about double what was seen in all other therapeutic OM3 hypertriglyceridemia trials. Several hypotheses are being investigated now by our clinical team, and by our CRO and Dr. Mozaffarian. These avenues of investigation are being carefully and rigorously pursued, and we are moving as quickly as possible to try to gain understanding and insight into the large and unexpected placebo response seen in TRILOGY 1. The Company will continue to provide updates on this investigation, as well as topline results for TRILOGY 2 as we get them, to be followed by all secondary and exploratory endpoints for TRILOGY 1 and 2 once the TRILOGY 2 study is completed and fully analyzed.”
Implementation of the TRILOGY 2 study remains on track, with the last patient having completed their final visit late last week. The Company remains blinded to the TRILOGY 2 data. Given the additional focus of critical resources now on TRILOGY 1, there could be a small delay of a couple weeks in reporting topline results for TRILOGY 2 to mid-February 2020. As previously disclosed, key secondary and exploratory endpoints from both studies, would be expected sometime later in the first quarter of 2020.
About CaPre
Acasti’s prescription drug candidate, CaPre, is a highly purified omega-3 phospholipid concentrate derived from krill oil, and is being developed to treat severe hypertriglyceridemia, a metabolic condition that contributes to increased risk of cardiovascular disease and pancreatitis. Its omega-3s, principally EPA and DHA, are either “free” or bound to phospholipids, which allows for better absorption into the body. Acasti believes that EPA and DHA are more efficiently transported by phospholipids sourced from krill oil than the EPA and DHA contained in fish oil that are transported either by triglycerides (as in dietary supplements) or as ethyl esters in other prescription omega-3 drugs, which must then undergo additional digestion before they are ready for transport in the bloodstream. Clinically, the phospholipids may not only improve the absorption, distribution, and metabolism of omega-3s, but they may also decrease the synthesis of LDL cholesterol in the liver, impede or block cholesterol absorption, and stimulate lipid secretion from bile. In two Phase 2 studies, CaPre achieved a statistically significant reduction of triglycerides and non-HDL cholesterol levels in patients across the dyslipidemia spectrum from patients with mild to moderate hypertriglyceridemia (patients with TG blood levels between 200mg/dl and 500mg/dl) to patients with severe hypertriglyceridemia (those with TG levels above 500mg/dl). Furthermore, in the Phase 2 studies, CaPre demonstrated the potential to actually reduce LDL, or “bad cholesterol”, as well as the potential to increase HDL, or “good cholesterol”, especially at the therapeutic dose of 4 grams/day. The Phase 2 data also showed a significant reduction of HbA1c at a 4 gram dose, suggesting that due to its unique omega-3/phospholipid composition, CaPre may actually improve long-term glucose metabolism. Acasti’s TRILOGY Phase 3 program is currently underway.
About Acasti Pharma
Acasti Pharma is a biopharmaceutical innovator advancing a potentially best-in-class cardiovascular drug, CaPre, for the treatment of hypertriglyceridemia, a chronic condition affecting an estimated one third of the U.S. population. Since its founding in 2008, Acasti Pharma has focused on addressing a critical market need for an effective, safe and well-absorbing omega-3 therapeutic that can make a positive impact on the major blood lipids associated with cardiovascular disease risk. The company is developing CaPre in a Phase 3 clinical program in patients with severe hypertriglyceridemia, a market that includes 3 to 4 million patients in the U.S. The addressable market may expand significantly if omega-3s demonstrate long-term cardiovascular benefits in on-going third party outcomes studies. Acasti may need to conduct at least one additional clinical trial to support FDA approval of a supplemental New Drug Application to expand CaPre’s indications to this segment. Acasti’s strategy is to commercialize CaPre in the U.S. and the company is pursuing development and distribution partnerships to market CaPre in major countries around the world. For more information, visit www.acastipharma.com.
Forward Looking Statements
Statements in this press release that are not statements of historical or current fact constitute “forward-looking information” within the meaning of Canadian securities laws and “forward-looking statements” within the meaning of U.S. federal securities laws (collectively, “forward-looking statements”). Such forward-looking statements involve known and unknown risks, uncertainties, and other unknown factors that could cause the actual results of Acasti to be materially different from historical results or from any future results expressed or implied by such forward-looking statements. In addition to statements which explicitly describe such risks and uncertainties, readers are urged to consider statements labeled with the terms “believes,” “belief,” “expects,” “intends,” “anticipates,” “potential,” “should,” “may,” “will,” “plans,” “continue”, “targeted” or other similar expressions to be uncertain and forward-looking. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this press release. Forward-looking statements in this press release include, but are not limited to, information or statements about Acasti’s strategy, future operations, prospects and the plans of management; Acasti’s ability to conduct all required clinical and non-clinical trials for CaPre, including the timing and results of those trials; the timing and the outcome of licensing negotiations; CaPre’s potential to become the “best-in-class” cardiovascular drug for treating severe Hypertriglyceridemia (HTG), Acasti’s ability to commercially launch CaPre and to fund its continued operations, CaPre’s potential to meet or exceed the target primary endpoint of reducing triglycerides by 20% compared to placebo, Acasti’s ability to report topline results for TRILOGY 2 in January 2020 as well as Acasti’s ability to report key secondary and exploratory endpoints from both TRILOGY studies by the end of the first quarter of 2020, and Acasti’s ability to file an NDA based on the TRILOGY studies.
The forward-looking statements contained in this press release are expressly qualified in their entirety by this cautionary statement, the “Cautionary Note Regarding Forward-Looking Information” section contained in Acasti’s latest annual report on Form 20-F and most recent management’s discussion and analysis (MD&A), which are available on SEDAR at www.sedar.com, on EDGAR at www.sec.gov/edgar/shtml, and on the investor section of Acasti’s website at www.acastipharma.com. All forward-looking statements in this press release are made as of the date of this press release. Acasti does not undertake to update any such forward-looking statements whether as a result of new information, future events or otherwise, except as required by law. The forward-looking statements contained herein are also subject generally to assumptions and risks and uncertainties that are described from time to time in Acasti’s public securities filings with the Securities and Exchange Commission and the Canadian securities commissions, including Acasti’s latest annual report on Form 20-F and most recent MD&A.
Neither NASDAQ, the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Acasti Contact:
Jan D’Alvise
Chief Executive Officer
Tel: 450-686-4555
Email: info@acastipharma.com
www.acastipharma.com
Investor Contact:
Crescendo Communications, LLC
Tel: 212-671-1020
Email: ACST@crescendo-ir.com