EXHIBIT 99.2
Published on May 7, 2021
Exhibit 99.2

1 Non - Confidential Information Presentation May 2021 DW1

Disclaimer This presentation does not constitute an offer to sell or the solicitation of an offer to buy any securities or a solicitation of any vote or approval . The proposed transaction will be submitted to the shareholders of Acasti Pharma Inc . (“Acasti”) for their consideration . Acasti will also prepare and file a Registration Statement on Form S - 4 that will include a prospectus/proxy statement for Acasti’s shareholders . Acasti plans to mail its shareholders a proxy statement in connection with the proposed transaction . Acasti may also file other documents with the Securities and Exchange Commission (the “SEC”) regarding the proposed transaction . INVESTORS AND SECURITYHOLDERS ARE URGED TO READ THE PROSPECTUS/PROXY STATEMENT AND ANY OTHER RELEVANT DOCUMENTS THAT WILL BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION . Investors and securityholders may obtain free copies of the prospectus/proxy statement and other documents containing important information about Acasti, Grace Therapeutics Inc . (“Grace”) and the proposed transaction once such documents are filed with the SEC through the website maintained by the SEC at www . sec . gov . Copies of the documents filed with the SEC by Acasti will be available free of charge on Acasti’s website at http : //www . acastipharma . com/ under the tab “Investor Relations” or by contacting Acasti by e - mail at ACST@crescendo - ir . com, or by phone at ( 450 ) 686 - 4555 . Acasti and Grace and certain of their respective directors and executive officers may be deemed to be participants in the solicitation of proxies from the shareholders of Acasti in connection with the proposed transaction . Information about the directors and executive officers of Acasti is set forth in Acasti’s definitive proxy statement for Acasti’s 2020 annual meeting of shareholders filed with the SEC on September 9 , 2020 . That document can be obtained free of charge from the sources indicated above . Additional information regarding the participants in the proxy solicitation and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the prospectus/proxy statement and other relevant materials to be filed with the SEC when they become available . 2

Forward - Looking Statements This presentation contains forward - looking statements, which are protected as forward - looking statements under the Private Securities Litigation Reform Act of 1995 , that are not limited to historical facts, but reflect Acasti’s and Grace’s current beliefs, expectations or intentions regarding future events . Words such as “may,” “will,” “could,” “should,” “expect,” “plan,” “project,” “intend,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “pursuant,” “target,” “continue,” and similar expressions are intended to identify such forward - looking statements . The statements in this presentation that are not historical statements, including statements regarding the expected timetable for completing the proposed transaction and benefits of the proposed transaction ; future product development plans and projected timelines for the initiation and completion of preclinical and clinical trials ; the potential for the results of ongoing preclinical or clinical trials and the efficacy of drug candidates ; the potential market opportunities and value of drug candidates ; other statements regarding future product development and regulatory strategies, including with respect to specific indications ; the tax treatment of the proposed transaction ; costs and other anticipated financial impacts of the proposed transaction ; the combined company’s plans, objectives, future opportunities, future financial performance and operating results ; sufficiency of capital resources to fund operating requirements ; and any other statements regarding Acasti’s and Grace’s future expectations, beliefs, plans, objectives, financial conditions, assumptions or future events or performance, are forward - looking statements within the meaning of the federal securities laws . These statements are subject to numerous risks and uncertainties, many of which are beyond Acasti’s or Grace’s control, which could cause actual results to differ materially from the results expressed or implied by the statements . These risks and uncertainties include, but are not limited to : failure to obtain the required votes or approvals of Acasti’s and/or Grace’s shareholders ; the timing to consummate the proposed transaction ; conditions to closing of the proposed transaction may not be satisfied or that the closing of the proposed transaction otherwise does not occur ; the risk that as a result of adjustments to the exchange ratio, Grace stockholders could own less of the combined company than is currently anticipated ; the risk that a regulatory approval that may be required for the proposed transaction is not obtained or is obtained subject to conditions that are not anticipated ; the diversion of management time on transaction - related issues ; the ultimate timing, outcome and results of integrating the operations of Acasti and Grace ; the effects of the business combination of Acasti and Grace following the consummation of the proposed transaction, including the combined company’s future financial condition, results of operations, strategy and plans ; the combined company’s need for, and the availability of, substantial capital in the future to fund its operations and research and development activities ; the combined company’s ability to continue to successfully progress research and development efforts and to create effective, commercially - viable products ; the success of the combined company’s product candidates in completing pre - clinical or clinical testing and being granted regulatory approval to be sold and marketed in the United States or elsewhere ; results of any litigation, settlements and investigations ; actions by third parties, including governmental agencies ; global economic conditions ; ability to effectively identify and enter new markets ; governmental regulations ; and ability to retain management and field personnel . Additional information concerning factors that could cause actual results to differ materially from those in the forward - looking statements is contained from time to time in Acasti’s SEC filings . Acasti’s filings may be obtained by contacting Acasti or the SEC or through Acasti’s web site at http : //www . acastipharma . com or through the SEC’s Electronic Data Gathering and Analysis Retrieval System (EDGAR) at http : //www . sec . gov . The foregoing list of risk factors is not exhaustive . These risks, as well as other risks associated with the proposed transaction will be more fully discussed in the prospectus/proxy statement that will be included in the Registration Statement on Form S - 4 that will be filed with the SEC in connection with the proposed transaction . Each of Acasti and Grace does not undertake any obligation to update or revise any forward - looking statements, whether as a result of new information, future events or otherwise, except as required by law . 3

Transaction Highlights » Acasti and Grace are a strong strategic fit: » Acasti gains a new pipeline of high potential clinical and preclinical programs » Acasti expands its expertise with Grace’s competencies in novel drug delivery technology and rare diseases » Combined company has significant drug development, regulatory and commercialization experience » First three clinical - stage assets from Grace have total US market potential of $850 million, with large Ex - US opportunity: » Each program addresses unmet medical needs with a highly differentiated value proposition for patients and providers » All assets granted Orphan Drug Designation (ODD) status, with potential for 7 - year US and 10 - year EU market exclusivity » Pursuing lower cost, lower risk 505(b)(2) regulatory pathway » Orphan drugs generally command premium pricing » Potential near - term, value - enhancing milestones for each product candidate » Approximately $64 million cash on hand expected to fund two lead assets through approval and pipeline advancement » Multiple additional preclinical drug candidates in pipeline for potential future development or partnering » Strong and growing intellectual property portfolio of >40 granted and pending patents in various global jurisdictions 4

About Grace Therapeutics » Leverage proprietary drug delivery capability and technologies to address rare and orphan diseases where there are significant unmet medical needs : » Focus on well - understood diseases that are poorly served by available therapies or have no approved therapies » Apply unique and proprietary drug delivery technologies to improve clinical outcomes, utilizing compounds proven to be safe » Provide better patient solutions with the potential to enhance efficacy with faster onset of action , reduce side effects , provide more convenient delivery , and increase patient compliance » Initially targeting three underserved orphan diseases - Subarachnoid Hemorrhage (SAH), Ataxia Telangiectasia (A - T), and Postherpetic Neuralgia (PHN) – all with sizable patient populations and significant market opportunity – estimated $850 million addressable market in the US alone » Potential for lower - risk , lower - cost pathway to drug development and commercialization: » Existence of well - defined clinical endpoints in target diseases that could facilitate faster clinical development and regulatory approval » Team has significant experience and successful track record in drug delivery technologies and clinical development to enhance product approval and commercialization: » Senior team collectively involved in the development and approval of several successful marketed drugs including ANDROGEL TM , SUBSYS TM , MARINOL TM and KEPPRA XR TM » Strong and growing IP portfolio with more than 40 granted and pending patents in various jurisdictions worldwide 5
Grace’s Lead Assets » GTX - 104: Subarachnoid Hemorrhage (SAH) – Intravenous Infusion » Disease Target : SAH is a rare and life - threatening medical emergency in which bleeding occurs over the surface of the brain in the subarachnoid space between the brain and skull » Product Description : Novel aqueous nanoparticle IV formulation of water insoluble nimodipine, which could improve the management of hypotension and vasospasm in SAH patients and potentially reduce the incidence of death and/or long - term disabilit y » GTX - 102: Ataxia - telangiectasia (A - T) – Oral Mucosal Spray » Disease Target : A - T is a progressive, neurodegenerative genetic disease that primarily impacts children causing severe disability, for which no treatment currently exists » Product Description : Novel, easy - to - use oral mucosal spray formulation of betamethasone intended to significantly improve neurological symptoms of A - T » GTX - 101: Postherpetic Neuralgia (PHN) – Topical Spray » Disease Target : PHN is a persistent and often debilitating neuropathic pain caused by nerve damage from the varicella zoster virus (shingles), which may persist for months and even years » Product Description : A novel bio - adhesive film forming topical spray for the treatment of postherpetic neuralgia, which could provide significant benefits over standard of care including greater convenience, and faster, longer onset of action 6
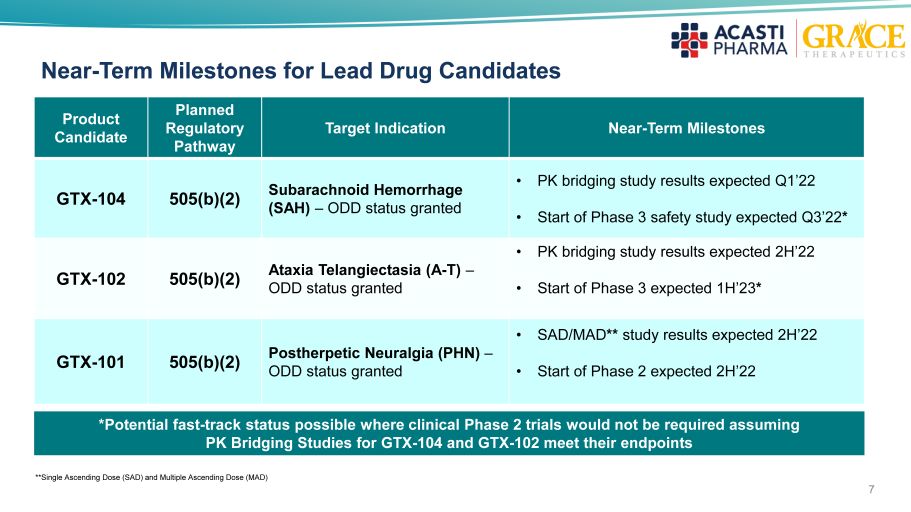
Product Candidate Planned Regulatory Pathway Target Indication Near - Term Milestones GTX - 104 505(b)(2) Subarachnoid Hemorrhage (SAH) – ODD status granted • PK bridging study results expected Q1’22 • Start of Phase 3 safety study expected Q3’22 * GTX - 102 505(b)(2) Ataxia Telangiectasia (A - T) – ODD status granted • PK b ridging study results expected 2H’22 • Start of Phase 3 expected 1H’23 * GTX - 101 505(b)(2) Postherpetic Neuralgia (PHN) – ODD status granted • SAD/MAD ** study results expected 2H’22 • Start of Phase 2 expected 2H’22 7 Near - Term Milestones for Lead Drug Candidates *Potential fast - track status possible where clinical Phase 2 trials would not be required assuming PK Bridging Studies for GTX - 104 and GTX - 102 meet their endpoints **Single Ascending Dose (SAD) and Multiple Ascending Dose (MAD)
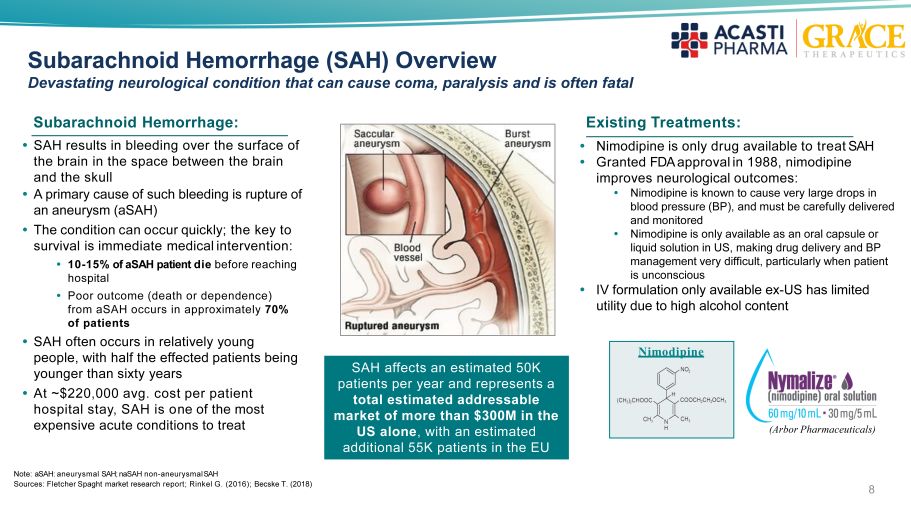
Subarachnoid Hemorrhage (SAH) Overview Devastating neurological condition that can cause coma, paralysis and is often fatal SAH results in bleeding over the surface of the brain in the space between the brain and the skull A primary cause of such bleeding is rupture of an aneurysm ( aSAH ) The condition can occur quickly ; the key to survival is immediate medical intervention : 10 - 15% of aSAH patient die before reaching hospital Poor outcome (death or dependence) from aSAH occurs in approximately 70% of patients SAH often occurs in relatively young people, with half the effected patients being younger than sixty years At ~$220,000 avg. cost per patient hospital stay, SAH is o ne of the most expensive acute conditions to treat Existing Treatment s: Nimodipine Nimodipine is only drug available to treat SAH Granted FDA approval in 1988, n imodipine improves neurological outcomes : Nimodipine is known to cause very large drops in blood pressure (BP), and must be carefully delivered and monitored Nimodipine is only available as an oral capsule or liquid solution in US, making drug delivery and BP management very difficult, particularly when patient is unconscious IV formulation only available ex - US has limited utility due to high alcohol content 37% 15% 37% 11% (Arbor Pharmaceuticals) SAH affects an estimated 50K patients per year and represents a total estimated addressable market of more than $300M in the US alone , with an estimated additional 55K patients in the EU Subarachnoid Hemorrhage: Note: aSAH: aneurysmal SAH; naSAH non - aneurysmal SA H Sources: Fletcher Spaght market research report; Rinkel G. (2016); Becske T. (2018) 8
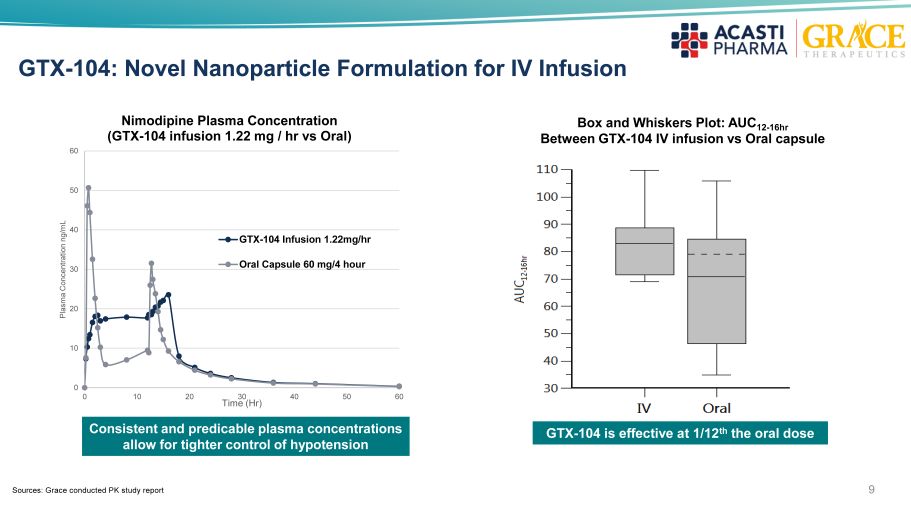
9 GTX - 104: Novel Nanoparticle Formulation for IV Infusion 0 10 20 30 40 50 60 0 10 20 30 40 50 60 Plasma Concentration ng/mL Time (Hr) Nimodipine Plasma Concentration (GTX - 104 infusion 1.22 mg / hr vs Oral) GTX-104 Infusion 1.22mg/hr Oral Capsule 60 mg/4 hour Box and Whiskers Plot: AUC 12 - 16hr Between GTX - 104 IV infusion vs Oral capsule Consistent and predicable plasma concentrations allow for tighter control of hypotension GTX - 104 is effective at 1/12 th the oral dose Sources: Grace conducted PK study report
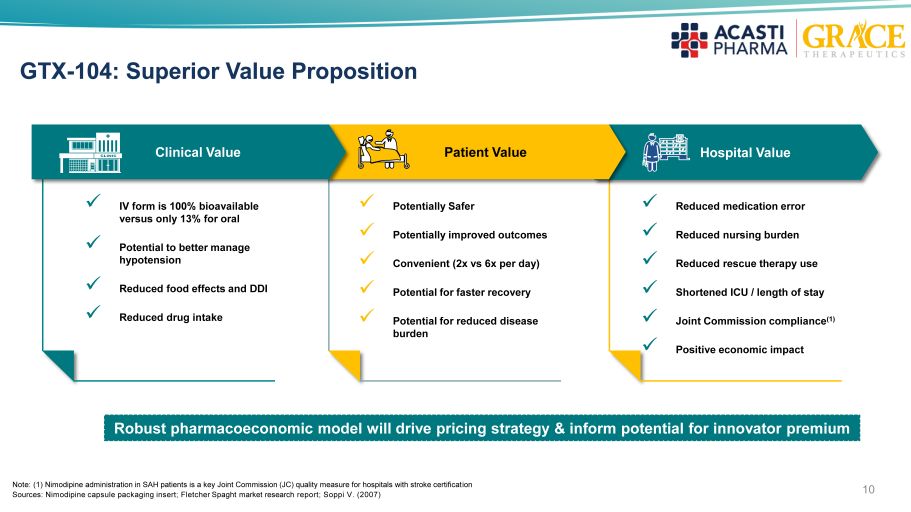
10 GTX - 104: Superior Value Proposition Hospital Value x IV form is 100% bioavailable versus only 13% for oral x Potential to better manage hypotension x Reduced food effects and DDI x Reduced drug intake x Potentially Safer x Potentially improved outcomes x Convenient (2x vs 6x per day) x Potential for faster recovery x Potential for reduced disease burden x Reduced medication error x Reduced nursing burden x Reduced rescue therapy use x Shortened ICU / length of stay x Joint Commission compliance (1) x Positive economic impact Patient Value Clinical Value Note: (1) Nimodipine administration in SAH patients is a key Joint Commission (JC) quality measure for hospitals with stroke certificat ion Sources: Nimodipine capsule packaging insert; Fletcher Spaght market research report; Soppi V. (2007) Robust pharmacoeconomic model will drive pricing strategy & inform potential for innovator premium
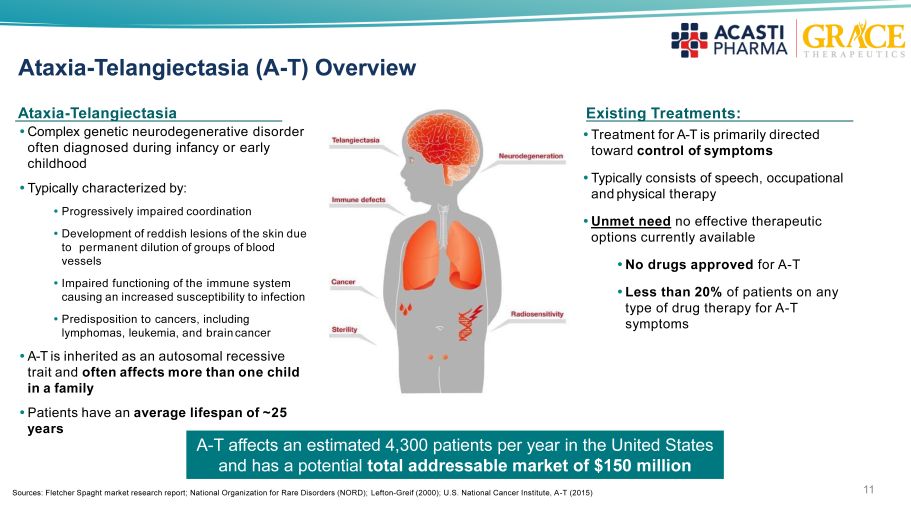
11 Ataxia - Telangiectasia (A - T) Overview Existing Treatment s: Ataxia - Telangiectasia Complex genetic neurodegenerative disorder often diagnosed during infancy or early childhood Typically characterized by: Progressively impaired coordination Development of reddish lesions of the skin due to permanent dilution of groups of blood vessels Impaired functioning of the immune system causing an increased susceptibility to infection Predisposition to cancers, including lymphomas, leukemia, and brain cancer A - T is inherited as an autosomal recessive trait and often affects more than one child in a family Patients have an average lifespan of ~25 years Treatment for A - T is primarily directed toward control of symptoms Typically consists of speech, occupational and physical therapy Unmet need no effective therapeutic options currently available No drugs approved for A - T Less than 20% of patients on any type of drug therapy for A - T symptoms A - T affects an estimated 4,300 patients per year in the United States and has a potential total addressable market of $150 million Sources: Fletcher Spaght market research report; National Organization for Rare Disorders (NORD); Lefton - Greif (2000); U.S. National Cancer Institute, A - T (2015)
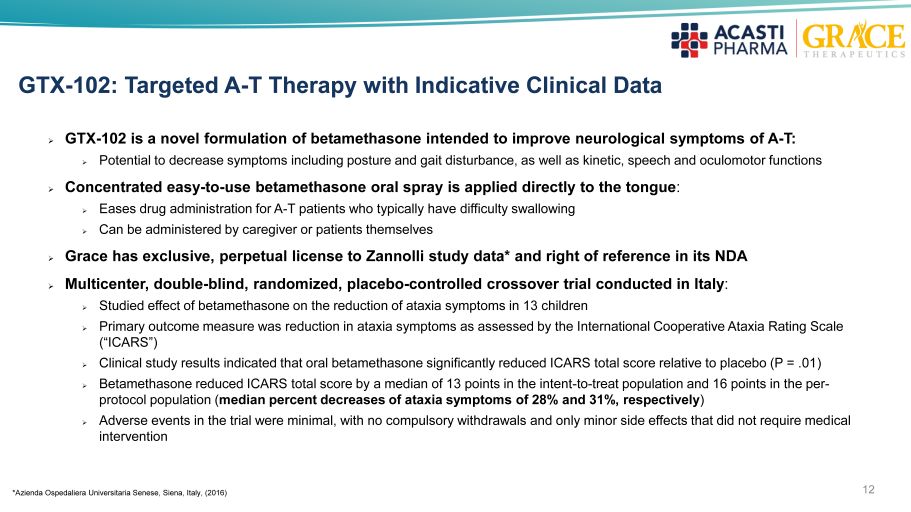
GTX - 102: Targeted A - T Therapy with Indicative Clinical Data » GTX - 102 is a novel formulation of betamethasone intended to improve neurological symptoms of A - T: » Potential to decrease symptoms including posture and gait disturbance, as well as kinetic, speech and oculomotor functions » Concentrated easy - to - use betamethasone oral spray is applied directly to the tongue : » Eases drug administration for A - T patients who typically have difficulty swallowing » Can be administered by caregiver or patients themselves » Grace has exclusive, perpetual license to Zannolli study data* and right of reference in its NDA » Multicenter, double - blind, randomized, placebo - controlled crossover trial conducted in Italy : » Studied effect of betamethasone on the reduction of ataxia symptoms in 13 children » Primary outcome measure was reduction in ataxia symptoms as assessed by the International Cooperative Ataxia Rating Scale (“ICARS”) » Clinical study results indicated that oral betamethasone significantly reduced ICARS total score relative to placebo (P = .01 ) » Betamethasone reduced ICARS total score by a median of 13 points in the intent - to - treat population and 16 points in the per - protocol population ( median percent decreases of ataxia symptoms of 28% and 31%, respectively ) » Adverse events in the trial were minimal, with no compulsory withdrawals and only minor side effects that did not require med ica l intervention 12 * Azienda Ospedaliera Universitaria Senese , Siena, Italy, (2016)
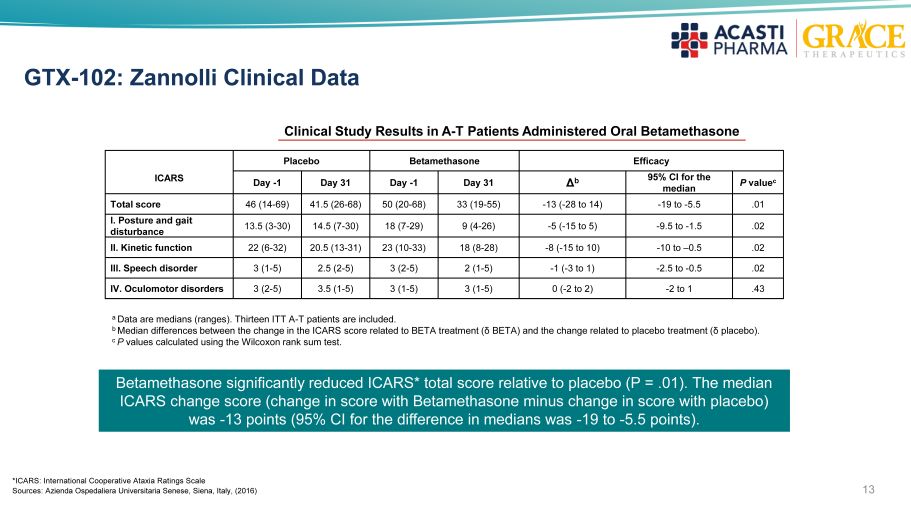
13 GTX - 102: Zannolli Clinical Data Clinical Study Results in A - T Patients Administered Oral Betamethasone Betamethasone significantly reduced ICARS* total score relative to placebo (P = .01). The median ICARS change score (change in score with Betamethasone minus change in score with placebo) was - 13 points (95% CI for the difference in medians was - 19 to - 5.5 points). ICARS Placebo Betamethasone Efficacy Day - 1 Day 31 Day - 1 Day 31 Δ b 95% CI for the median P value c Total score 46 (14 - 69) 41.5 (26 - 68) 50 (20 - 68) 33 (19 - 55) - 13 ( - 28 to 14) - 19 to - 5.5 .01 l. Posture and gait disturbance 13.5 (3 - 30) 14.5 (7 - 30) 18 (7 - 29) 9 (4 - 26) - 5 ( - 15 to 5) - 9.5 to - 1.5 .02 ll. Kinetic function 22 (6 - 32) 20.5 (13 - 31) 23 (10 - 33) 18 (8 - 28) - 8 ( - 15 to 10) - 10 to – 0.5 .02 lll. Speech disorder 3 (1 - 5) 2.5 (2 - 5) 3 (2 - 5) 2 (1 - 5) - 1 ( - 3 to 1) - 2.5 to - 0.5 .02 lV. Oculomotor disorders 3 (2 - 5) 3.5 (1 - 5) 3 (1 - 5) 3 (1 - 5) 0 ( - 2 to 2) - 2 to 1 .43 a Data are medians (ranges). Thirteen ITT A - T patients are included. b Median differences between the change in the ICARS score related to BETA treatment (δ BETA) and the change related to placebo tr eatment (δ placebo). c P values calculated using the Wilcoxon rank sum test. *ICARS: International Cooperative Ataxia Ratings Scale Sources: Azienda Ospedaliera Universitaria Senese , Siena, Italy, (2016)
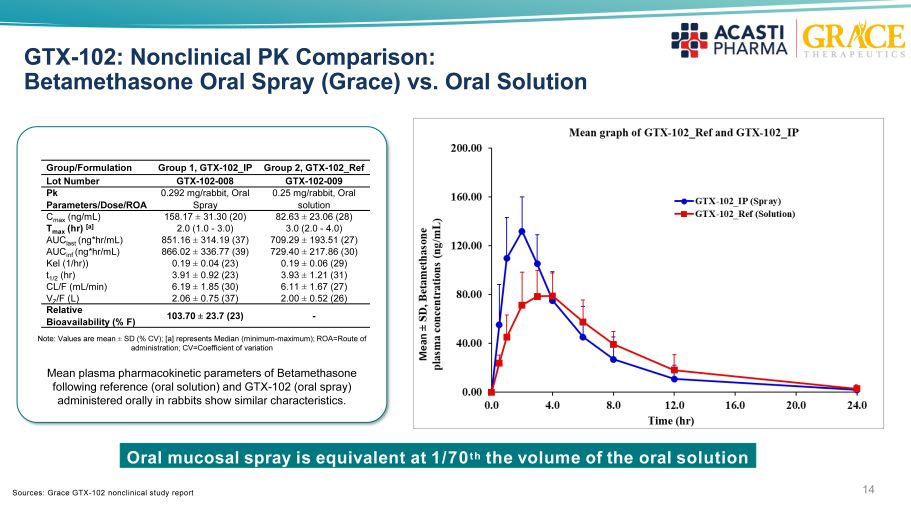
14 GTX - 102: Nonclinical PK Comparison: Betamethasone Oral Spray (Grace) vs. Oral Solution Or al mucosal spray is equivalent at 1/70 th the volume of the o ral solution Note: Values are mean ± SD (% CV); [a] represents Median (minimum - maximum); ROA=Route of administration; CV=Coefficient of variation Mean plasma pharmacokinetic parameters of Betamethasone following reference (oral solution) and GTX - 102 (oral spray) administered orally in rabbits show similar characteristics. Group/Formulation Group 1, GTX - 102_IP Group 2, GTX - 102_Ref Lot Number GTX - 102 - 008 GTX - 102 - 009 Pk Parameters/Dose/ROA 0.292 mg/rabbit, Oral Spray 0.25 mg/rabbit, Oral solution C max (ng/mL) 158.17 ± 31.30 (20) 82.63 ± 23.06 (28) T max (hr) [a] 2.0 (1.0 - 3.0) 3.0 (2.0 - 4.0) AUC last (ng*hr/mL) 851.16 ± 314.19 (37) 709.29 ± 193.51 (27) AUC inf (ng*hr/mL) 866.02 ± 336.77 (39) 729.40 ± 217.86 (30) Kel (1/hr)) 0.19 ± 0.04 (23) 0.19 ± 0.06 (29) t 1/2 (hr) 3.91 ± 0.92 (23) 3.93 ± 1.21 (31) CL/F (mL/min) 6.19 ± 1.85 (30) 6.11 ± 1.67 (27) V Z /F (L) 2.06 ± 0.75 (37) 2.00 ± 0.52 (26) Relative Bioavailability (% F) 103.70 ± 23.7 (23) - Sources: Grace GTX - 102 nonclinical study report
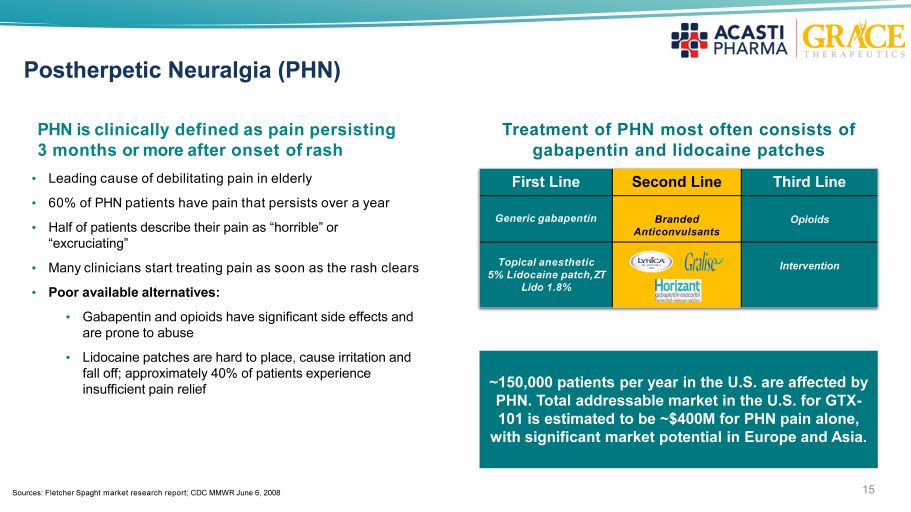
15 Postherpetic Neuralgia (PHN) PHN is clinically defined as pain persisting 3 months or more after onset of rash • Leading cause of debilitating pain in elderly • 60% of PHN patients have pain that persists over a year • Half of patients describe their pain as “horrible” or “excruciating” • Many clinicians start treating pain as soon as the rash clears • Poor available alternatives: • Gabapentin and opioids have significant side effects and are prone to abuse • Lidocaine patches are hard to place, cause irritation and fall off; approximately 40% of patients experience insufficient pain relief First Line Second Line Third Line Generic gabapentin Branded Anticonvulsants Opioids Topical anesthetic 5% Lidocaine patch, ZT Lido 1.8% Intervention Treatment of PHN most often consists of gabapentin and lidocaine patches ~150,000 patients per year in the U.S. are affected by PHN. Total addressable market in the U.S. for GTX - 101 is estimated to be ~$400M for PHN pain alone, with significant market potential in Europe and Asia. Sources: Fletcher Spaght market research report; CDC MMWR June 6, 2008
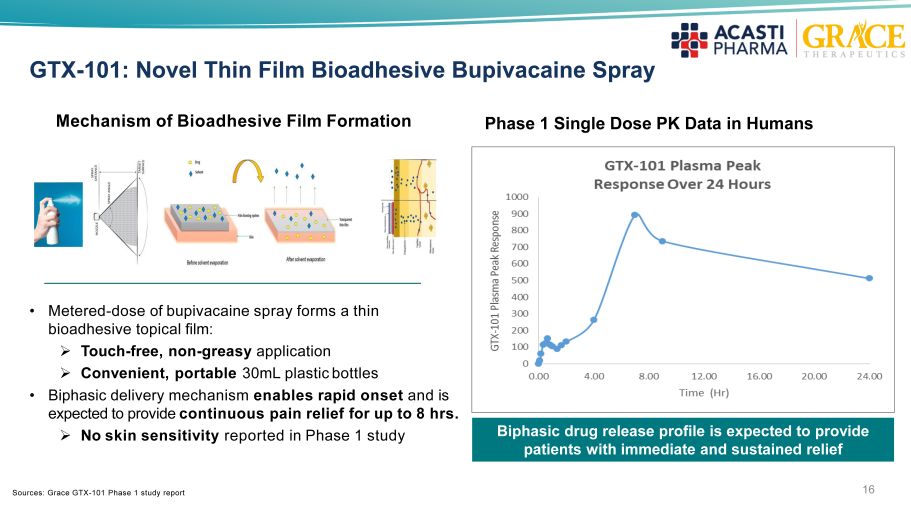
16 GTX - 101: Novel Thin Film Bioadhesive Bupivacaine Spray • Metered - dose of bupivacaine spray forms a thin bioadhesive topical film : » Touch - free, non - greasy application » Convenient, portable 30mL plastic bottles • Biphasic delivery mechanism enables rapid onset and is expected to provide continuous pain relief for up to 8 hrs . » No skin sensitivity reported in Phase 1 study Mechanism of Bioadhesive Film Formation Biphasic drug release profile is expected to provide patients with immediate and sustained relief Phase 1 Single Dose PK Data in Humans Sources: Grace GTX - 101 Phase 1 study report
Easier, more convenient application • Potential for rapid onset of action and extended relief • Easy to use (no peeling and cutting patches to size) • Ability to precisely cover the affected area • No skin sensitivity or irritation in Phase 1 trial • More potent analgesic and opioid - sparing • Well understood and efficacious • Longer acting vs. lidocaine • Strong KOL support for bupivacaine Bupivacaine, an ideal topical analgesic • Convenience will improve compliance and expand use • Potential future market for non - PHN pain indications • Enthusiasm across specialties Use for PHN and potentially other indications 17 GTX - 101: Superior Value Proposition Sources: Fletcher Spaght market research report

18 Transaction Structure and Financial Highlights » Structure: Acquisition of Grace by Acasti via merger in a 100% stock transaction, with Grace becoming a direct wholly - owned subsidiary of Acasti » Ownership: Acasti shareholders owning 55% and Grace stockholders owning 45% of the combined company (on a fully diluted basis), subject to upward adjustments in favor of Acasti based on closing net cash positions and capitalizations of Acasti and Grace » Leadership: Jan D'Alvise will continue as CEO, Brian Ford as CFO, Pierre Lemieux and George Kottayil (Grace) will co - lead all R&D activities and Prashant Kohli (Grace) will lead commercial operations » Board Representation: Combined board will consist of four Acasti designees and three Grace designees » Target Cash Balance: Combined Company is expected to have unrestricted cash of over $64 million, assuming closing in Q3, 2021 » Support of Grace board and stockholders: » Grace board and stockholders have approved the transaction » Major shareholders have agreed to a post - closing lockup for one year » Financing Contingency: None » Closing Conditions: Customary conditions, including shareholder and NASDAQ / TSXV approvals » Timing: Closing Q3, 2021 subject to SEC review and shareholder approvals Source: Acquisition Agreement and Plan of Merger, dated May 6, 2021, among Acasti, Grace and Acasti’s wholly - owned subsidiary

19 Experienced Acasti/Grace Leadership Team Pierre Lemieux, Ph.D. COO & CSO, Acasti Jan D’Alvise President & CEO, Acasti Prashant Kohli VP, Commercial Operations, Grace George Kottayil, Ph.D. CEO and Co - Founder of Grace Brian Ford CFO, Acasti

20 Grace Board of Directors Name & Title Biography Experience Vimal Kavuru, RPh Chairman & Co - Founder • Founder, Chairman & CEO of Rising Pharma Holdings, Inc., one of the top 20 US generic pharmaceutical companies. Previously, Mr. Kavuru was Founder of Citron Pharma & Lucid Pharma which were sold to Aceto Corporation 2016. • Founder of Casper Pharma LLC, an emerging specialty brand pharmaceutical company and Chairman of Cronus Pharma LLC, an animal health pharmaceutical company. • Co - founded Celon Labs, Sequoia Capital funded specialty oncology and critical care pharma in India recently acquired by Zanzibar Pharma, a portfolio company of CDC Group, UK’s development finance institution. Founded Gen - Source RX, a national distributor of generic pharmaceuticals acquired by Cardinal in 2014. • Registered Pharmacist, BS, Pharmacy, HKE College of Pharmacy, India William Haseltine, PhD Director • Dr. Haseltine is Chairman & President of ACCESS Health International, Inc. and Chairman of the Haseltine Foundation for Science and the Arts. He is the Founder of Human Genome Sciences and served as its Chairman and CEO for twelve years. He is also the founder of several other successful biotechnology companies. Eight pharmaceutical products from companies that he started are currently approved by US and international regulatory agencies. • He is a Founder and CEO of Demetrix , Inc., a biotechnology company specializing in pain and anxiety medications, and a Founder and Director of X - VAX, which is developing a novel Herpes Simplex vaccine. He was a professor at Harvard Medical School and Harvard School of Public Health, where he was the Founder and Chair of the Division of Biochemical Pharmacology and the Division of Human Retrovirology. He is well known for his seminal work on cancer, HIV/AIDS and genomics, and has authored more than 200 manuscripts published in peer reviewed journals. • Dr. Haseltine is a lifetime member of the New York Academy of Sciences, a Trustee of the New York Academy of Medicine, and an honorary trustee of the Brookings Institution.

Contact Information Acasti Contact: Jan D’Alvise Chief Executive Officer Tel: 450 - 686 - 4555 Email: info@acastipharma.com www.acastipharma.com Investor Contact: Crescendo Communications, LLC Tel: 212 - 671 - 1020 Email: ACST@crescendo - ir.com 21